Thiopurine Methyltransferase Activity
Thiopurine drugs include Azathioprine (Imuran), 6-mercaptopurine (Purinethol, 6-MP) and 6-thioguanine. They are used to treat acute lymphoblastic leukemia and a variety of autoimmune diseases, such as Crohn disease and rheumatoid arthritis, and organ transplant recipients. The parent drugs are prodrugs that are metabolized to 6-thioguanine nucleotides. The cytotoxic effect of these drugs is achieved by incorporation of 6-thioguanine into DNA and of leukocytes, resulting in DNA/RNA damage, cell death and myelosuppression. This step is necessary for their antimetabolite activity and therapeutic efficacy.
The enzyme, thiopurine methyltransferase (TPMT), provides a competitive pathway for inactivation of these drugs by thiol methylation. A balance must be established between these 2 competing metabolic pathways such that sufficient drug is converted to 6-thiguanine to act as an antimetabolite, but the level does not become so high as to cause lethal bone marrow suppression.
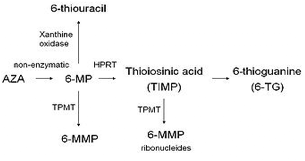
TPMT activity is inherited as a monogenic codominant trait. Distinct inheritable differences in levels of red blood cell TPMT have been detected among patients. Patients with low or absent TPMT activity metabolize the drugs more slowly and have higher thioguanine nucleotide concentrations, increasing the risk of developing severe, life-threatening myelosupression if conventional doses are given.
Approximately 0.3% of the population has undetectable TPMT activity, 11% low (intermediate) activity, and 89% normal activity. Between 30 and 60% of individuals with intermediate TPMT activity cannot tolerate a full thiopurine dose. They should be prescribed a 30 to 70% reduction in thiopurine dose. Homozygous individuals with undetectable TPMT activity require approximately 90% reduction in thiopurine dose or treatment with an alternative medication. Patients with higher than normal TPMT activity may appear to be therapeutic failures because they metabolize thioguanine nucleotides too quickly.
Ideally, TPMT activity should be determined before prescribing immediate full doses of a thiopurine. If therapy cannot be delayed, immediate full doses can be administered and the dose can be adjusted based on TPMT results. This strategy is permissable because most patients do not reach steady-state concentrations of thioguanine nucleotide metabolites for 2 to 6 weeks.
TPMT phenotyping quantitates TPMT enzyme activity in erythrocytes. The activity of TPMT is measured as the nanomoles of 6-methylmercaptopurine (inactive metabolite) produced per 1 mL of packed red blood cells and expressed as U/mL. Reference ranges at ARUP Laboratories are:
| TPMT Level | Interpretation |
| 24.0-44.0 U/mL RBC | Normal TPMT activity |
| 17.0-23.9 U/mL RBC | Intermediate activity |
| 0 - 17.0 U/mL RBC | Low TPMT activity |
Numerous patients have values which are near the cutoff between normal and the heterozygous state due to both assay variability and biological variation.
TPMT enzyme activity can be inhibited by many drugs including, but not limited to: naproxen, ibuprofen furosemide, sulfasalazine, mefenamic acid, thiazide diuretics, and benzoic acid inhibitors. TPMT phenotyping should not be performed for at least 90 days after a red blood cell transfusion. If testing cannot be delayed, TPMT genotyping can be performed.
The allele for normal (wild-type) TPMT activity has been designated TPMT*1. Four TPMT alleles, TPMT2, 3A, 3B, and 3C, account for over 90% of inactivating polymorphisms. Therefore, most reference laboratories only test for these genetic variants. Based on the result, a predicted phenotype of normal, intermediate, or low TPMT activity is assigned.
| TPMT Genotype | Predicted Genotype |
| 1/1 | Normal activity |
| 1/2, 1/3A, 1/3B, 1/3C | Intermediate activity |
|
2/2, 2/3A, 2/3B, 3A/3C 3A/3A, 3A/3B, 3A/3C 3B/3B, 3B/3C 3C/3C |
Low or absent activity |
Less frequent causes of TPMT deficiency are TPMT alleles TPMT4, TPMT5, TPMT8, and TPMT12.
Genotyping is not affected by medications known to inhibit TPMT phenotyping. TPMT genotyping should not be performed in patients who have undergone allogeneic bone marrow transplantation, since the result would reflect the donor's genotype, not the patient.
The US Food and Drug Administration, the Clinical Pharmacogenetics Implementation Consortium, and some professional societies recommend consideration of TPMT genotype or TPMT erythrocyte testing prior to the initiation of therapy with thiopurine drugs.
Specimen requirement is one 5 mL green-top (heparin) tube of whole blood. Specimen must be refrigerated and tested within 72 hours of draw.
References
Appell ML, Berg J, Duley J, et al: Nomenclature for alleles of the thiopurine methyltransferase gene. Pharmacogenet Genomics 2013;23(4):242-248
DiPiero J, Teng K, Hicks JK. Should TPMT activity be determined before prescribing azathioprine, mercaptopurine, or thioguanine? Cleveland Clin J Med 2015;82 (7): 409-13.


